INSPIRATM ART 100


revolutionizes extracorporeal blood circulation by integrating cutting-edge
technology with intuitive design to optimize patient care during crucial times.
On-screen patient monitoring

In-Depth Incident Analysis
promoting continuous learning and driving quality
improvements within your team.

Alarm Troubleshooting Tips
level of anxiety*.
Red Book”, 6th edition, chapter 7 - Circuit Malfunction and Crisis Management
On-screen procedure overview
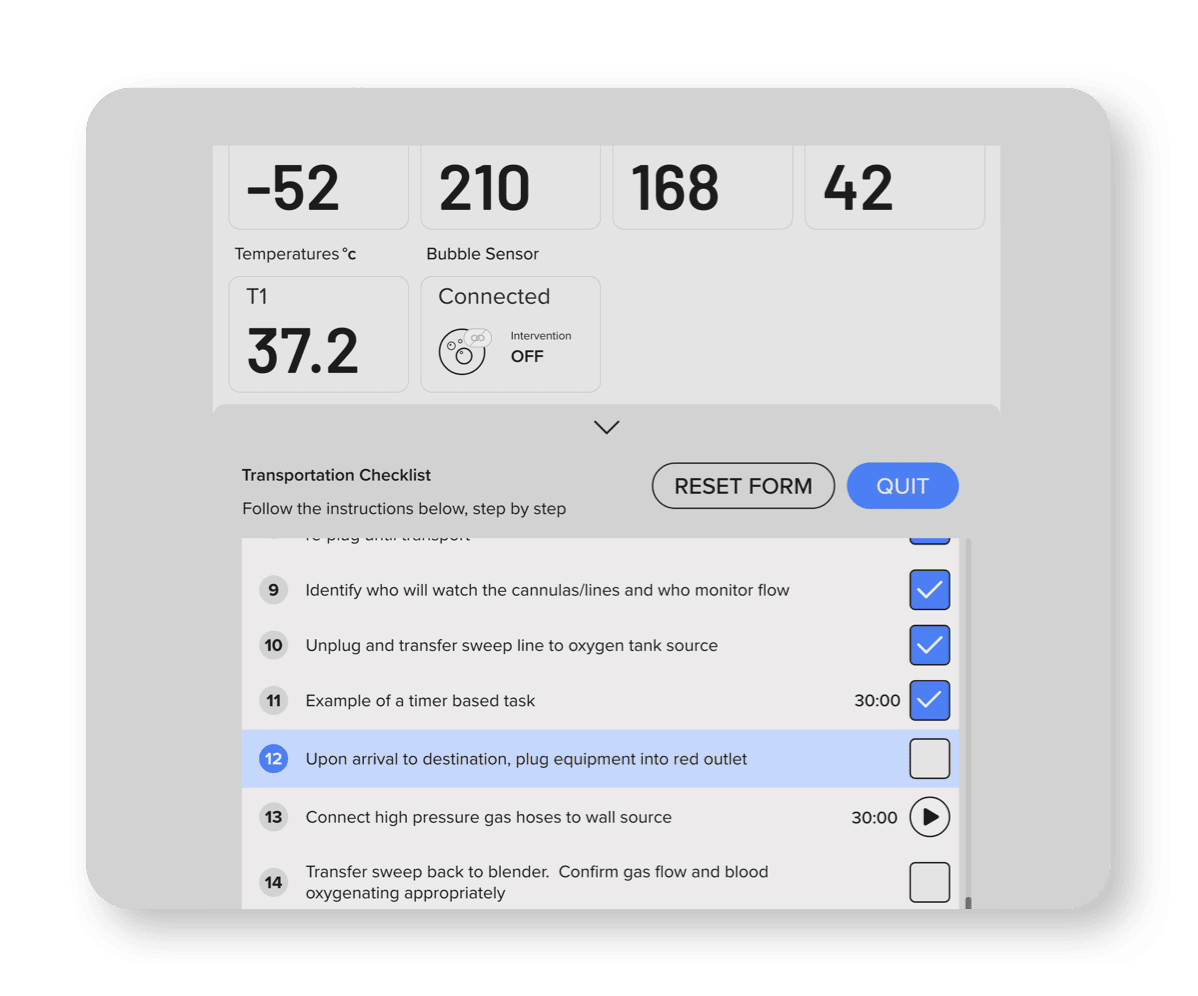
Hospital-Customizable Checklists
step aligns with your practice routines and is
executed precisely.

Comprehensive Procedure Overview
and alarms, to enhance team communication and
facilitate smooth information sharing.

Your solution
Portability
Features a compact design, foldable handle, and up to 4 hours of battery life.
24/7 Operational Support
Our dedicated support team is available to ensure continuous smooth operations, with quick spare parts delivery, minimizing downtime.
Your Flexibility in Choice
Compatible with various disposable parts, allows for flexible customization of tubing and oxygenators, which simplifies purchasing and inventory management.



Future Capabilities
Clip-on HYLA™ blood sensor real-time blood monitoring
The clip-on HYLA™ blood sensor aims to alert physicians
of changes in a patient’s condition without the need for
intermittent actual blood samples. This real-time,
continuous monitoring technology could be applicable
in extracorporeal blood oxygenation procedures.